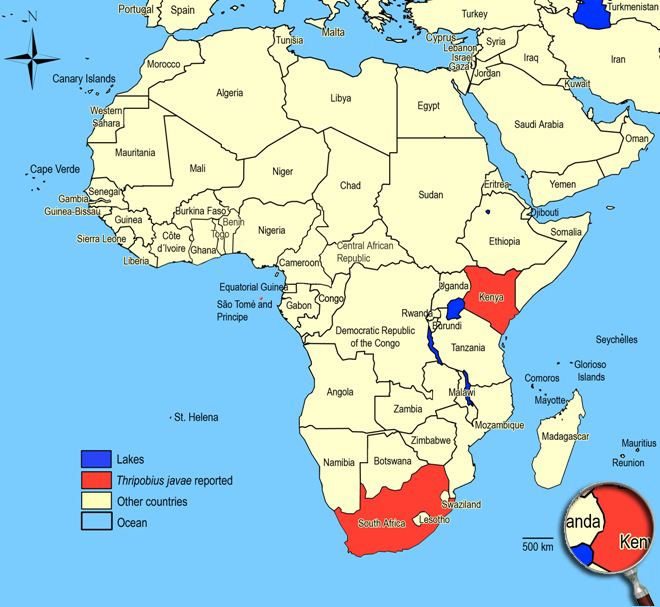
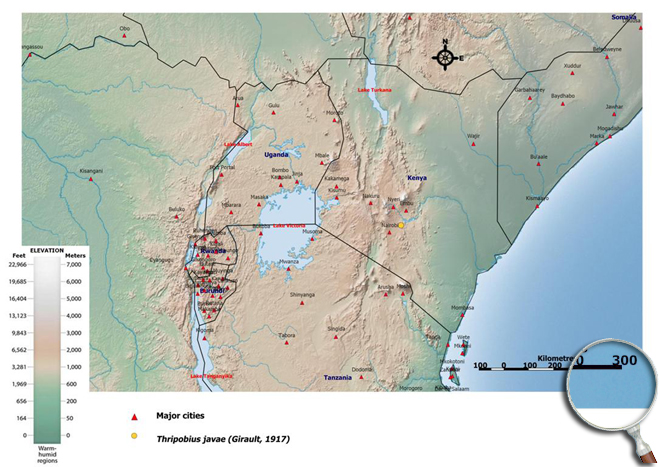
Beattie GAC & Jaing L (1990). Greenhouse thrips and its parasitoids in coastal New South Wales. General and Applied Entomology. 22: 21-24
Bernardo U, Iodice L, Sasso R & Pedata PA (2008). Effects of cold storage on Thripobius javae (= T. semiluteus) (Hymenoptera: Eulophidae). Biocontrol Science and Technology. 18 (9): 921-933
Bernardo U, Viggiani G. & Sasso R (2005). Biological parameters of Thripobius semiluteus Bouček (Hym., Eulophidae), a larval endoparasitoid of Heliothrips haemorrhoidalis (Bouché) (Thysan., Thripidae). Journal of Applied Entomology. 129 (5): 250-257
Bouček Z (1976). Taxonomic notes on some Eulophidae [Hym.] of economic interest, mainly from Africa. Entomophaga. 21 (4): 401-414
Bouček Z (1988). Australasian Chalcidoidea (Hymenoptera): a biosystematic revision of genera of fourteen families, with a reclassification of species. CAB International, Wallingford, Oxon, UK, 832 pp
Chiu HT (1984). The ecology and chemical control of grape-vine thrips (Rhipiphorothrips cruentatus Hood) on wax apple. Plant Protection Bulletin (Taiwan). 26 (4): 365-378
Ferrière C (1938). Descriptions of some African Eulophidae (Hym. Chalc.). Bulletin of Entomological Research. 29 (2): 141-147
Froud KJ & Stevens PS (1997). Life table comparison between the parasitoid Thripobius semiluteus and its host greenhouse thrips. Proceedings of the Fiftieth New Zealand Plant Protection Conference, Lincoln University, Canterbury, New Zealand, 18-21 August 1997, pp. 232-235
Froud KJ & Stevens PS (2002). Importation biological control of Heliothrips haemorrhoidalis by Thripobius semiluteus in New Zealand - A case study of non-target host and environmental risk assessment. Proceedings of the 1st International Symposium on Biological Control of Arthropods, Honolulu, Hawaii, 14-18 January 2002, pp. 366-369 http://www.invasiveforestinsectandweedbiocontrol.info/international_symposium/day5_pdf/froud.pdf
Froud KJ & Stevens PS (2004). Estimating the host range of a thrips parasitoid, pp. 90-102. In van Driesche RG, Murray T & Reardon R [eds.], Assessing host ranges for parasitoids and predators used for classical biological control: A guide to best practice. USDA, Forest Health Technology Enterprise Team, Morgantown, West Virginia
Froud KJ, Stevens PS & Cowley DR (1996). A potential biological control agent for greenhouse thrips. Proceedings of the Forty Ninth New Zealand Plant Protection Conference, Quality Hotel Rutherford, Nelson, New Zealand, 13-15 August 1996, pp.17-20. New Zealand Plant Protection Society, Rotorua, New Zealand
Gibson, G.A.P., Read, J.D. & Fairchild, R. 1998. Chalcid wasps (Chalcidoidea): illustrated glossary of positional and morphological terms
Girault AA (1917). New Javanese Hymenoptera. Privately published, Washington, D.C., 1 p
Goodwin S & Steiner MY (1996). Survey of Australian native natural enemies for control of thrips. IOBC/WPRS Bulletin. 19 (1): 47-50
Herting B (1971). Arachnida to Heteroptera. A catalogue of parasites and predators of terrestrial arthropods. Section A. Host or Prey / Enemy, 1. Commonwealth Agricultural Bureaux, Slough, England, 129 pp
Husain T & Khan MY (1986). Family Eulophidae. In Subba Rao BR & Hayat M [eds.], The Chalcidoidea (Insecta: Hymenoptera) of India and the adjacent countries. Part II. Catalogues. Oriental Insects. 20: 211-245
Jamieson LE, Froud KJ, Edwards R & Stevens PS (2008). Establishment of Thripobius javae (= semiluteus) in New Zealand. New Zealand Plant Protection. 61: 17-23
La Salle J & McMurtry JA (1989). First record of Thripobius semiluteus (Hymenoptera: Eulophidae) from the New World. Proceedings of the Entomological Society of Washington. 91 (4): 634
Loomans AJM (1991). Collection and first evaluation of hymenopterous parasites of thrips as biological control agents of Frankliniella occidentalis. IOBC/WPRS Bulletin. 14 (5): 73-82
Loomans AJM, Murai T & Green ID (1997). Interactions with hymenopterous parasitoids and parasitic nematodes, pp. 355-397. In Lewis T [ed.], Thrips as crop pests. CAB International, Wallingford, Oxon, UK
Loomans AJM & van Lenteren JC (1995). Biological control of thrips pests: a review on thrips parasitoids, pp. 89-201. In Loomans AJM, van Lenteren JC, Tommasini MG, Maini S & Riudavets J [eds.], Biological control of thrips pests. Wageningen Agricultural University Papers, 95-1. Veenman Drukkers, Wageningen, The Netherland
McMurtry JA (1988). Biological control on greenhouse thrips. Citrograph. 73 (4): 81-82
McMurtry JA & Badii MH (1991). Greenhouse thrips, Heliothrips haemorrhoidalis, in California avocado orchards: biological control studies, pp. 393-398. In Parker BL, Skinner M & Lewis T [eds.], Towards understanding Thysanoptera. Proceedings International Conference on Thrips, Burlington, Vermont, USA, February 21-23 1989. General Technical Report NE-147. USDA, Forest Service, North Eastern Forest Experiment Station
McMurtry JA, Johnson HG & Newberger SJ (1991). Imported parasite of greenhouse thrips established on California avocado. California Agriculture. 45 (6): 31-32
Mills NJ (1991). Thrips biocontrol: opportunities for use of natural enemies against the pear thrips, pp. 373-391. In Parker BL, Skinner M & Lewis T [eds.], Towards understanding Thysanoptera. Proceedings International Conference on Thrips, Burlington, Vermont, USA, February 21-23, 1989. General Technical Report NE-147. USDA, Forest Service, North Eastern Forest Experiment Station
Mineo G, Sinacori A & Viggiani G (1999). Introduction of Thripobius semiluteus Boucek (Hymenoptera, Eulophidae) for the biological control of the greenhouse thrips Heliothrips haemorrhoidalis (Bouche) (Thysanoptera, Thripidae). Phytophaga, Palermo. 9 (supplemento): 13-20
Mohan Daniel A (1986). Thrips-parasite interactions in some panchaetothripine Thysanoptera (Insecta: Arthropoda). Proceedings of the Indian National Science Academy, Part B, Biological Sciences. 52 (4): 437-444
Newberger SJ & McMurty JA (1992). Insectary production of Thripobius semiluteus Boucek (Hymenoptera: Eulophidae), an important parasitoid of the greenhouse thrips, Heliothrips haemorrhoidalis (Bouché). International Report, University of California at Riverside, 3 pp
Noyes JS (2002). Interactive catalogue of world Chalcidoidea 2001. The Natural History Museum, Taxapad 2002, CD-ROM
Ritchie AH (1932). Report of the entomologist, 1931. Annual Report. Tanganyika Territory, Department of Agriculture. 1931: 83-86
Ritchie AH (1933). Report of the entomologist, 1932. Annual Report. Tanganyika Territory, Department of Agriculture. 1932: 68-72
Schauff ME (1991). The Holarctic genera of Entedoninae (Hymenoptera: Eulophidae). Contributions of the American Entomological Institute. 26 (4): 1-109
Siddapaji C & Reddy DNRN (1974). A new record of Ceranisus sp. (Eulophidae: Hymenoptera) as a parasite of Panchaetothrips indicus Bagnall, (Heliothripinae - Thysanoptera) in cardamom. Current Research. 3 (10): 125-126
Sinacori A, Mineo N & Mineo G (2002). First records on Thripobius semiluteus Boucek (Hymenoptera: Eulophidae) in Sicily. Bollettino del Laboratorio di Entomologia Agraria 'Filippo Silvestri', Portici. 57: 89-91
Steyn WP (1996). First record of Thripobius semiluteus Boucek (Hymenoptera: Eulophidae) as a parasitoid of pine-tree thrips nymphs in South Africa. Information Bulletin, Institute for Tropical and Subtropical Crops, Agricultural Research Council. 289: 14-16
Steyn WP, du Toit WJ & de Beer M (1993a). Parasitoid of the pine-tree thrips recorded in SA. Information Bulletin, Institute for Tropical and Subtropical Crops, Agricultural Research Council. 250: 1-2
Steyn WP, du Toit WJ & de Beer M (1993b). Natural enemies of thrips on avocado. Yearbook, South African Avocado Growers' Association. 16: 105-106
Steyn WP, du Toit WJ & de Beer M (1993c). Thripobius semiluteus (Eulophidae), a potential biocontrol agent of Heliothrips haemorrhoidalis and Selenothrips rubrocinctus (Thripidae) on avocado. Proceedings of the 9th Entomology Congress, ESSA, Johannesburg, 160 pp
Thompson WR (1955). A catalogue of the parasites and predators of insect pests. Section 2. Host parasite catalogue, Part 3. Hosts of the Hymenoptera (calliceratid to evaniid). Commonwealth Agricultural Bureaux, The Commonwealth Institute of Biological Control, Ottawa, Ontario, Canada
Triapitsyn SV (2005). Revision of Ceranisus and the related thrips-attacking entedonine genera (Hymenoptera: Eulophidae) of the world. African Invertebrates. 46: 261-315
Triapitsyn SV & Headrick DH (1995). A review of the Nearctic species of the thrips-attacking genus Ceranisus Walker (Hymenoptera: Eulophidae). Transactions of the American Entomological Society. 121 (4): 227-248
Viggiani G, Bernardo U & Sasso R (2000). First results on the introduction of Thripobius semiluteus Boucek (Hymenoptera: Eulophidae) into Italy for the biological control of Heliothrips haemorrhoidalis (Bouché) (Thysanoptera). Atti Giornate Fitopatologiche Perugia, 16-20 aprile 2000. 1: 521-526
Waterston J (1930). Two new parasitic Hymenoptera. The Annals and Magazine of Natural History, Zoology, Botany, and Geology. (Series 10) 5: 243-246
Wysoki M, Kuzlitzky W, Ishar Y, Swirski E, Ben-Yehuda S, Hadar D & Reneh S (1997). Successful acclimatization of Thripobius semiluteus, a
parasitoid of Heliothrips haemorrhoidalis (Bouché) in Israel. Phytoparasitica. 25 (2): 155